by Gary Ciment, Ph.D., Scientific Director, Aves Labs, Inc.
Cochlin is a 53kDa secreted glycoprotein and a member of the integrin superfamily of gene products (Bhattacharya, 2006). Although its function is largely unknown, its primary amino acid structure contains a pair of Von Willebrand Factor domains (i.e., glycoprotein binding domains), which may explain its presence within extracellular matrices (Figure 1). Cochlin is expressed and secreted by a wide variety of different cell types, including cells of the inner ear and ciliary epithelial cells of the anterior chamber of the eye.
In the anterior chamber of the eye, ciliary epithelial cells produce intraocular fluid (IOF) which plays a direct role in maintaining overall eye shape and an indirect role in maintaining optic nerve health. After being produced by ciliary epithelial cells, IOF flows through various regions of the anterior chamber, eventually leaving this chamber through a trabecular meshwork adjacent to the canal of Schlemm, where it is reabsorbed by a capillary bed. The rates of IOF production and reabsorption need to be carefully balanced in order to maintain proper intraocular pressure (IOP). In this sense, the trabecular meshwork plays a key role in maintaining this balance by controlling hydrostatic resistance to fluid flow -- too much resistance caused by the trabecular meshwork leads to the dangerous buildup of IOP within the eye, which in turn, leads to optic nerve pathology and eventually blindness. The medical term used to describe the pathological buildup of excessive IOP is glaucoma, and in most cases, is believed to be the direct result of dysregulation of flow resistance through the trabecular meshwork (Sommer, 1989; Lütgen-Drecoll, 2000).
In 2005, Dr. Sanjoy Bhattacharya approached Aves Labs with the goal of producing a set of custom antibodies that would allow his group to study the possible role of cochlin in regulating IOP and contributing to glaucoma. Cochlin had previously been shown to be involved in various unrelated genetic disorders, including those involved in blood coagulation (from which cochlin received its name -- COagulation factor C Homology) and a particular form of late-onset deafness called DFNA9 (Robertson et al., 1997). Intriguingly, these DFNA9 patients not only presented with the progressive high frequency hearing loss, but also with glaucoma.
From the amino acid sequence of human cochlin that Dr. Bhattacharya sent us, we looked for appropriate peptide sequences using our proprietary Immunogenicity Algorithm®. From the list of peptide candidates, Dr. Bhattacharya chose three for making antipeptide antibodies -- #1 from a region between the LCCL domain and the first von Willebrandt Factor A domain, and #2 and #3 from within the second von Willebrandt Factor A domain (Figure 1). These peptides were chosen in part because they were distant to the two known N-glycosylation sites in cochlin (at asparagines 100 and 221), which would certainly have abrogated the ability of antibodies raised against non-glycosylated peptides to recognize the native protein. In general, it is important to avoid peptides that correspond to regions of target proteins that contain sites of post-translational modifications, such as phosphorylations and glycosylations.

Figure 1. Primary amino acid structure of the human precursor of cochlin (adapted from Piciani et al., 2007)
After consultantion with Dr. Bhattacharya, we added a cysteine and a synthetic spacer amino acid to the N-terminus of these sequences, and synthesized the peptide. A keyhole limpet hemocyanin (KLH) conjugate of these peptides were injected over a 7-week period into the breast muscles of six laying hens (a pair of hens for each peptide), and then eggs were collected over a 3-week period after the 4th injection. From 12 of these eggs from each pair of hens, we purified several grams of IgY using our proprietary methods, and from this IgY preparation we further purified dozens of milligrams of affinity purified antibodies. Since the hens were unharmed during the egg collection phase of the project, we were able to perform additional injections and collect additional eggs.
With these affinity-purified antibodies, Dr. Bhattacharya's group began to look at the relationship between cochlin and glaucoma. Figure 2 shows the trabecular meshwork of the trabecular meshwork in two age- and sex-matched human donors. Panel A is a non-glaucomatous 36-year old male donor, whereas panel b shows a 34-year old male donor who had been suffering with glaucoma. Note the presence of cochlin-immunoreactivity in the trabecular meshwork of the glaucomatous donor, but not the control donor (Picciani et al., 2007; see also Bhattacharya et al., 2005; Wang et al., 2015). Western blots of trabecular meshwork homogenates prepared from other donors confirm that the immunoreactivity seen in Figure 2 corresponded to cochlin protein (Figure 3) (Picciani et al., 2007). These studies show that cochlin localization within the trabecular meshwork is indeed correlated with glaucoma, but it doesn't necessarily show cause-and-effect.

Figure 2. Cochlin immunoreactivity in the trabecular meshwork of glaucomatous (upper and lower panels b), but not control donors (upper and lower panels a). Note the presence of cochlin-immunoreactivity (red staining) in the lower panel b (from a glaucomatous donor), but not in the lower panel a (from a non-glaucomatous donor. This immunoreactivity was produced by affinity-purified cochlin #3 antibodies produced at Aves Labs. The asterisks in all of the panels shows the location of the Canal of Schlemm as an anatomical reference point. (from Picciani et al., 2007)

Figure 3. Western blots showing the presence of cochlin-immunoreactivity in trabecular meshwork homogenates from glaucomatous, but not non-glaucomatous donors. Lane a is a loading control of collagen II (using a rabbit anti-collagen type II antibody); lane d uses affinity-purified anti-cochlin peptide antibodies (cochlin#1) prepared at Aves Labs. Note the presence of cochlin immunoreactivity at the correct molecular weight (53 kDa) in the trabecular meshwork from the glaucomatous donors, but not non-glaucomatous donors. (from Picciani et al., 2007)
Using a mouse model of glaucoma, Dr. Bhattacharya's group then began a series of studies using the cochlin cDNA inserted into a lentivirus vector in order to cause overexpression of cochlin. DBA mice normally do not develop IOP or glaucoma with age. However, when lentivirus vectors containing the cochlin gene under the control of constitutive promoter was injected intraocularly into DBA mice, their IOP increased about 2-fold within a couple of days, and remained elevated for weeks (Goel et al., 2012) (see also Figure 4A). Immunohistochemical studies using Aves Labs' antibodies confirmed the presence of cochlin immunoreactivity in the trabecular meshwork (Goel et al., 2012). Uninjected mice or mice injected with a negative control gene, human serum albumin, did not exhibit significant changes in IOP.
To test whether underexpression of cochlin would cause decreased IOP, mice were injected with another lentivirus causing expression of a short hairpin loop anti-sense RNA (shRNA, also known as "silencing RNA"). In these studies, injection with the cochlin shRNA caused a decrease in IOP within 10 days, and this decrease persisted for months (Goel et al., 2012) (see also Figure 4B).
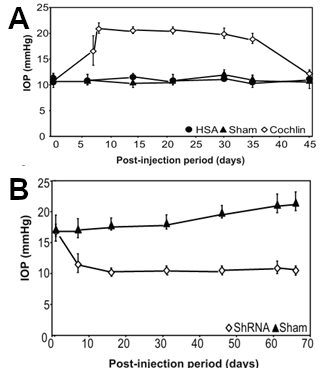
Figure 4. Overexpression of cochlin-GFP fusion protein increases IOP in mice, whereas expression of a cochlin-silencing RNA decreases IOP.
In Panel A, mice were injected intraocularly with a lentivirus containing either a cochlin-GFP construct to cause overexpression of a cochlin fusion protein (open diamonds) or a human serum albumin (HSA) fusion protein (closed circles) or sham-injected closed triangles, and then IOP was measured after various periods of time. Note that injection of the cochlin fusion protein resulted in a significant increase in IOP as early as 5 days post-injection.
In Panel B, mice were injected intraocularly with a lentivirus containing a short hairpin silencing RNA (ShRNA) (open diamonds), or sham injected (closed triangles), and their IOP was measured after various periods of time. Note that injection of the silencing RNA resulted in a significant decrease in IOP, as compared to sham-injected mice.
(adapted from Goel et al., 2012)
In more recent work, Dr. Bhattacharya has studied the molecular mechanisms by which cochlin and various cochlin-binding proteins such as TREK-1 and Annexin A2 may increase flow resistance within the trabecular meshwork (Goel et al., 2011; Carreon et al., 2017). These studies illustrate the complex mechanisms by which IOF flow through the trabecular meshwork is regulated, and how dysregulation can contribute to glaucoma and its consequences, such as blindness.
References
- Bhattacharya, S.K. (2006). Focus on Molecules: Cochlin. Exp. Eye Res. 82(3): 355-356.
- Bhattacharya, S.K., Rockwood, E.J., Smith, S.D., Bonilha, V.L., Crabb, J.S., Kuckey, R.W., Robertson, N.G., Peachey, N.S., Morton, C.C., Crabb, J.W. (2005). Proteomics Reveals Cochlin Deposits Associated with Glaucomatous Trabecular Meshwork. J. Biol. Chem. 280: 6080-6084.
- Carreon, T.A., Castellanos, A., Gasuli, X., Bhattacharya, S.K. (2017). Interaction of Cochlin and Mechanosensitive Channel TREK-1 in Trabecular Meshwork Cells Influences the Regulation of Intraocular Pressure. Sci. Rep. 7: 452; doi:10.1038/s41598-017-00430-2
- Goel, M., Sienkiewicz, A.E., Picciani, R., Lee, R.K., Bhattacharya, S.K. (2011). Cochlin Induced TREK-1 Co-Expression and Annexin A2 Secretion: Role in Trabecular Meshwork Cell Elongation and Motility. PLoS ONE 6(8): e23070. doi:10.1371/journal.pone.0023070
- Goel, M., Slenkiewicz, A.E., Picciani, R., Wang, J., Lee, R.K., Bhattacharya, S.K. (2012). Cochlin, Intraocular Pressure Regulation and Mechanosensing. PLoS ONE 7(4): e34309. doi:10.1371/journal.pone.0034309.
- Lütjen-Drecoll, E. (2000). Important of Trabecular Meshwork Changes in the Pathogenesis of Primary Open Angle Glaucoma. J. Glaucoma 9: 417-418.
- Picciani, R., Sesai, K., Guduric-Fuchs, J., Cogliati, T., Morton, C.C., Bhattacharya, S.K. (2007). Cochlin in the eye: Functional Implications. Prog. Retin. Eye Res. 26(5): 433-469.
- Robertson, N.G., Skvorak, A.B., Yin, Y., Weremowicz, S., Johnson, K.R., Kovatch, K.A., Battey, J.F., Bieber, F.R., Morton, C.C. (1997). Mapping and Characterization of a Novel Cochlear Gene in Human and in Mouse: A positional Candidate Gene for a Deafness Disorder, DRNA9. Genomics 46: 345-354.
- Sommer, A. (1989). Intraocular Pressure and Glaucoma. Amer. J. Ophthal. 107(2): 186-188.
- Wang, J., Aljohani, A., Carreon, T., Gregori, G., Bhattacharya, S.K. (2015). In vivo quantitication of cochlin in glaucomatous DBA/2J mice using optical coherence tomography. Sci. Rep. 5, 11092; doi:10.1038/srep 11092.

