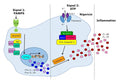
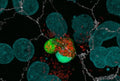
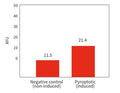
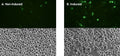
Ships: 1-2 business days
- Prepare samples and controls.
- Dilute 10X Cellular Assay Buffer 1:10 with diH2O.
- Reconstitute FAM-FLICA with 50 µL DMSO.
- Dilute FAM-FLICA 1:5 by adding 200 µL PBS.
- Add diluted FAM-FLICA to each sample at 1:30-1:60 (e.g., spike at 1:30 by adding 10 µL to 290 µL sample).
- Incubate approximately 1 hour.
- Remove media and wash cells 3 times: add 1X Cellular Wash Buffer and spin cells.
- If desired, label with additional stains, such as Hoechst 33342, Propidium Iodide, 7-AAD, or an antibody.
- If desired, fix cells.
- Analyze with a fluorescence microscope, fluorescence plate reader, or flow cytometer. FAM-FLICA excited at 492 nm and emits at 520 nm.
Kit 9145: 25-50 Tests
FLICA Caspase-1 Inhibitor Reagent (FAM-YVAD-FMK), 1 vial, #655
10X Cellular Wash Buffer, 15 mL, #6164
Fixative, 6 mL, #636
Hoechst 33342, 1 mL, #639
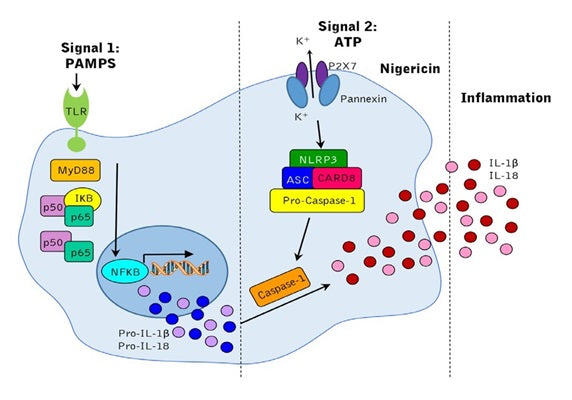
Nigericin, 0.5 µmoles, #6698
Kit Manual
Kit 9146: 100-200 Tests
FLICA Caspase-1 Inhibitor Reagent (FAM-YVAD-FMK), 4 vials, #655
10X Cellular Wash Buffer, 60 mL, #6165
Fixative, 6 mL, #636
Hoechst 33342, 1 mL, #639
Nigericin, 0.5 µmoles, #6698
Kit Manual
Product Specific References
| PMID | Publication |
| 39513885 | Lien, T, et al. 2024. Targeted Delivery to Dying Cells Through P-Selectin-PSGL-1 Axis: A Promising Strategy for Enhanced Drug Efficacy in Liver Injury Models. Cells, 1778. |
| 39169447 | Liang, Z., et al. 2024. Activation of the HMGB1-TLR4 pathway impacts the functionality of bone marrow mesenchymal stem cells and disrupts macrophage polarization in immune thrombocytopenia. British Journal of Haematology, . |
| 38936048 | Jia, ZC, et al. 2024. Chlorogenic acid can improve spermatogenic dysfunction in rats with varicocele by regulating mitochondrial homeostasis and inhibiting the activation of NLRP3 inflammasomes by oxidative mitochondrial DNA and cGAS/STING pathway. Bioorganic Chemistry, 107571. |
| 38791301 | Munalisa, R., et al. 2024. Restraint Stress-Induced Neutrophil Inflammation Contributes to Concurrent Gastrointestinal Injury in Mice. International journal of molecular sciences. |
| 38652514 | Wang, J, et al. 2024. Egr1 promotes Nlrc4-dependent neuronal pyroptosis through phlda1 in an in-vitro model of intracerebral hemorrhage. Neuroreport. |
| 37807970 | Liu, S.J., et al. 2023. Ursolic acid alleviates chronic prostatitis via regulating NLRP3 inflammasome-mediated Caspase-1/GSDMD pyroptosis pathway. Phytotherapy research: PTR, . |
| 37890377 | Lu, Y., et al. 2023. HDL inhibits pancreatic acinar cell NLRP3 inflammasome activation and protect against acinar cell pyroptosis in acute pancreatitis. International immunopharmacology, 110950. |
| 37373116 | Kim, D., et al. 2023. Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression Via Downregulation of EzH2. SSRN Electronic Journal, 9968. |
| 37224982 | Dong, R.J., et al. 2023. Thalidomide promotes NLRP3/caspase-1-mediated pyroptosis of macrophages in Talaromyces marneffei infection. Microbial pathogenesis, 106168. |
| 37629059 | Li, C.C., et al. 2023. Restraint Stress-Induced Immunosuppression Is Associated with Concurrent Macrophage Pyroptosis Cell Death in Mice. International journal of molecular sciences. |
| 35126599 | Liu, M., et al. 2022. Zhilong Huoxue Tongyu Capsule Alleviated the Pyroptosis of Vascular Endothelial Cells Induced by ox-LDL through miR-30b-5p/NLRP3. Evidence-based complementary and alternative medicine : eCAM, 3981350. |
| 35444640 | Hung, S.C., et al. 2022. Nanodiamond-Induced Thrombocytopenia in Mice Involve P-Selectin-Dependent Nlrp3 Inflammasome-Mediated Platelet Aggregation, Pyroptosis and Apoptosis. Frontiers in immunology, 806686. |
| 35836796 | Xiong, J., et al. 2022. DUSP2-mediated inhibition of tubular epithelial cell pyroptosis confers nephroprotection in acute kidney injury. Theranostics, 5069-5085. |
| 35758658 | He, S., et al. 2022. PRRSV Infection Induces Gasdermin D-Driven Pyroptosis of Porcine Alveolar Macrophages through NLRP3 Inflammasome Activation. Journal of virology, e0212721. |
| 36009329 | Martino, E., et al. 2022. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants. |
| 35967457 | Su, M., et al. 2022. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nature cardiovascular research, 732-747. |
| 36069386 | Tian, J., et al. 2022. Calycosin represses AIM2 inflammasome-mediated inflammation and pyroptosis to attenuate monosodium urate-induced gouty arthritis through NF-κB and p62-Keap1 pathways. Drug development research. |
| 36181338 | Yang, Z., et al. 2022. TREM-1 induces pyroptosis in cardiomyocytes by activating NLRP3 inflammasome through the SMC4/NEMO pathway. The FEBS journal. |
| 36394994 | Wang, Y.H., et al. 2022. Discovery of a Series of 5-Amide-1H-pyrazole-3-carboxyl Derivatives as Potent P2Y14R Antagonists with Anti-Inflammatory Characters. Journal of medicinal chemistry. |
| 33436548 | Chen, A., et al. 2021. Rosuvastatin protects against coronary microembolization-induced cardiac injury via inhibiting NLRP3 inflammasome activation. Cell death & disease, 78. |